Properties of Colloidal Solutions
Properties of Colloidal Solutions: Overview
This topic covers concepts such as Properties of Colloidal Solutions, Size of Colloidal Particles, Viscosity and Surface Tension of Colloidal Solutions, VIsibility of Colloidal Solutions, Filterability of Colloidal Solutions, etc.
Important Questions on Properties of Colloidal Solutions
Choose the option that is depicting the correct match:
(i) KCl, an electrolyte, is added to hydrated ferric oxide sol a) Tyndall effect
(ii) an electric current is passed through a colloidal solution b) electrophoresis
(iii) a beam of light is passed through a colloidal solution. c) mutual coagulation
Among the electrolytes the most effective coagulating agent for sol is:
How coagulation can be carried out by mixing two oppositely charged sols?
Describe briefly any three methods by which coagulation of lyophobic sols can be carried out.
Describe any two methods of coagulation.
Viscosity of lyophilic colloidal solution is same as that of dispersion medium.
Which is the following is/are correct for lyophilic sols?
When milk is cooled, the protein particles coagulate and start floating on the surface.
Which particles float on the surface, when milk is cooled?
When milk is cooled, the _____ particles coagulate and start floating on the surface.
Give an example of coagulation by cooling.
How many of the following mixtures show Tyndall effect?
Ferric hydroxide
in benzene
urea
starch
Isoelectric point refers to the ion concentration at which, the colloidal particles
Colloidal solution . Osmotic pressure of and are found to be higher and lower than , respectively. Then
Volume of a colloidal particle, as compared to the volume of a solute particle in a true solution, could be
of are added to of solution. The ions which will move towards the cathode and anode, respectively, are
The zig-zag movement of particles suspended in liquids and gases is called _____ named after the scientist _____.
Which is true for all types of colloids?
The Brownian movement is due to
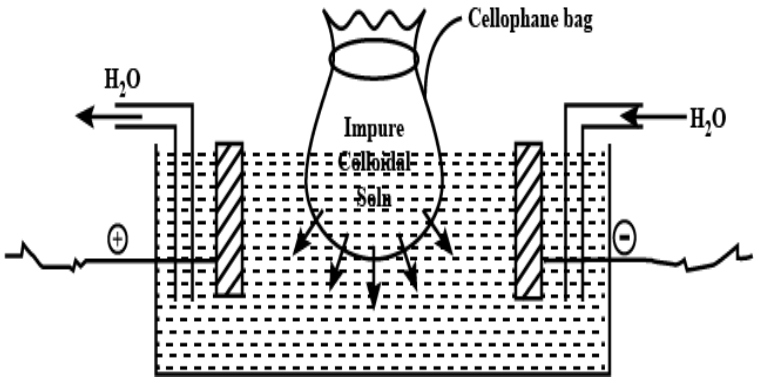
Name of the proces involved in the above diagram:
